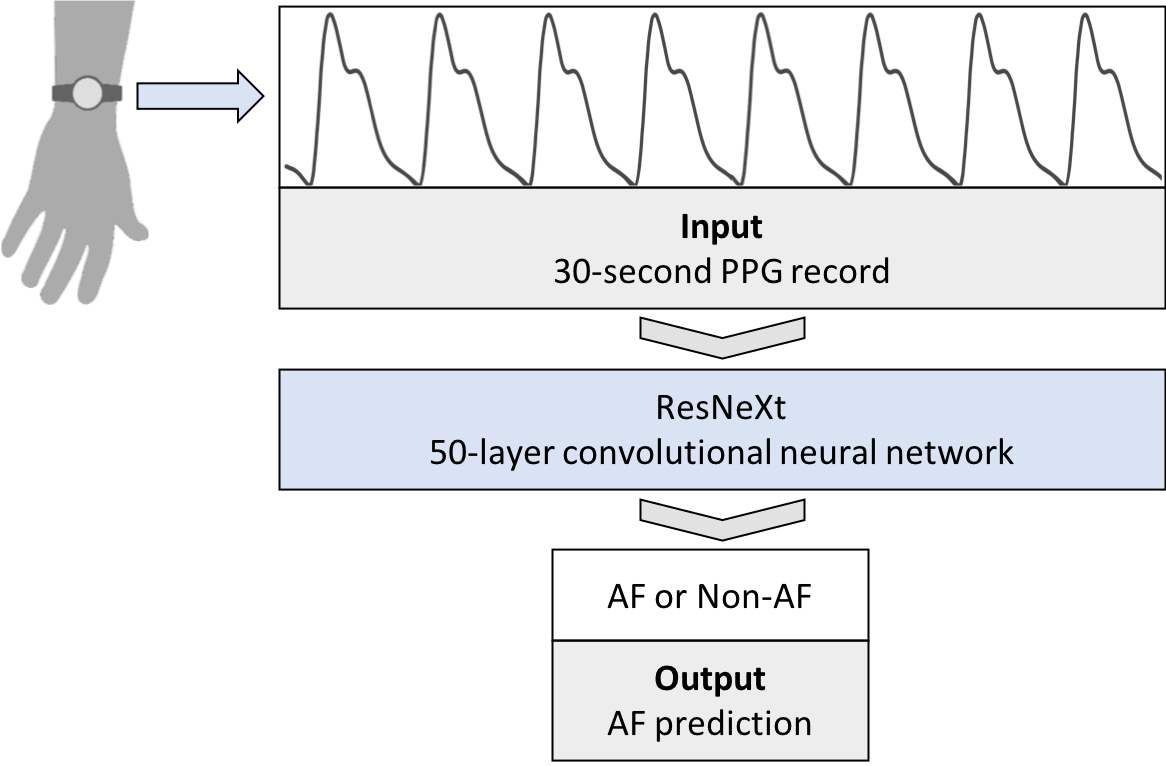
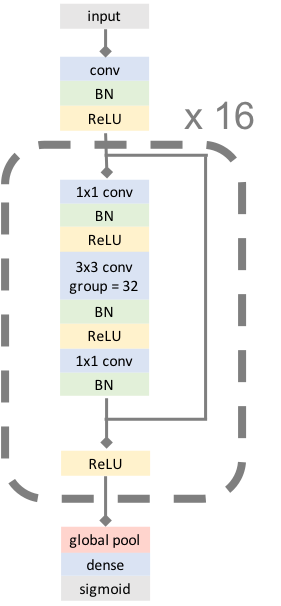
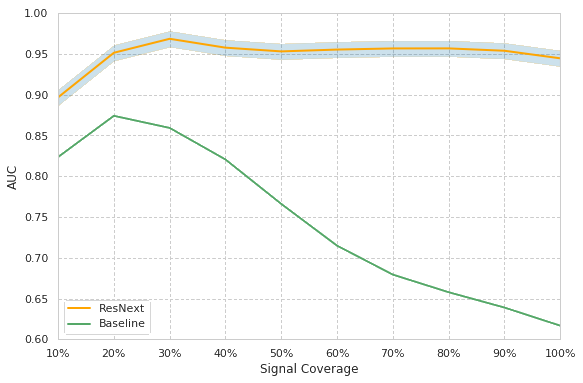
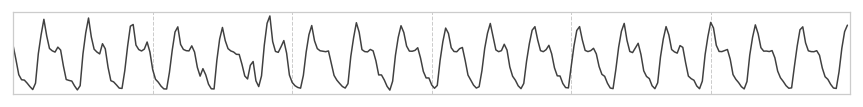
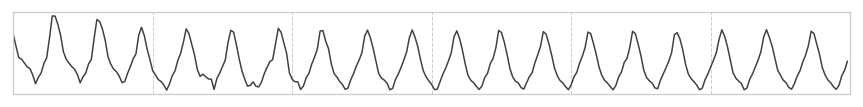
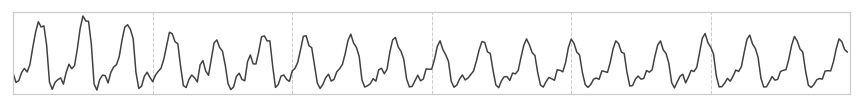
We develop an algorithm which accurately detects Atrial Fibrillation (AF) episodes from photoplethysmograms (PPG) recorded in ambulatory free-living conditions, with 95% AUC.
Atrial fibrillation (AF) is the most common cardiac arrhythmia and a leading risk factor for stroke, affecting at least 2.7 million adults in the United States. The diagnosis is usually performed by observing electrocardiograms (ECG) typically measured with a cardiac event recorder, a Holter monitor or a chest patch. However, these ECG devices tend to be used in a reactive manner. Many occurrences of subclinical or silent AF are thus undetected.
Photoplethysmography (PPG) is an emerging technology that enables non-invasive heart rhythm measurement through optical sensing. A PPG sensor detects blood volume changes in the microvascular bed of tissue using a low intensity light. Nowadays, many existing wearable devices on the market have built-in PPG sensors. Continuous and accurate detection of AF from PPG has the potential to transform low-cost consumer wearable devices into clinically useful medical monitoring tools in mass scale.
In this work, we present the first model to continuously and accurately detect AF episodes in PPG collected in an ambulatory free-living setting.
Read our paper